Core Service
What does MediRama do?
We are Clinical Development & Commercialization Expert Group
We provide a one-stop, consolidated clinical development & commercialization service (ABCD Platform)

Clinical/Regulatory Consulting
- Clinical Strategy
- : Indication prioritization, non-clinical gap analysis, accelerated approval planning, TPP, 1a/1b synopsis, protocol development
- Regulatory Strategy & Engagement
- - FDA: ODD, FTD, BTD
- - MFDS: ODD
- - Regulatory Engagement: FDA/MFDS Pre-IND/IND & Type B, C, D meetings
- - IND/NDA Support: Approval-driven regulatory strategy, gap analysis, CMC/non-clinical/clinical evaluation
- Advisory Committee

CRO & Trial Management
- CRO Services
- : IND, startup, monitoring, PV, data management/statistics,
medical writing and logistics
- Trial Excellence
- : Sponsor-side study team, site/investigator selection & management, regulatory interaction, CRO oversight, medical monitoring, DSUR/IB update, IDMC/DSMB operation
- Patient & Risk Management
- : Investigator meetings, recruitment strategy, risk assessment & mitigation,
response-type analysis for protocol refinement
- Quality Control
- My MD & My PM

Commercial Consulting
- Corporate Portfolio & Business Strategy
- Licensing Deals for Pipeline Products
- - Business development consulting (BD strategy, partnering, valuation,
negotiation, counteract and term sheet)
- Business Strategy for Local Market
- - Market access, launch, distribution, promotion, PR, alliance, etc.
Clinical & Regulatory Consulting

From clinical development stage, MediRama can help you with
- Comprehensive clinical development strategy: Identifying unmet needs, selecting clinical indications
- Clinical trial operation/management: Selecting and overseeing clinical vendors and investigators, communication with regulatory authorities
Drug candidate development up to the preclinical stage
Platform development
Target identification
Lead optimization
Preclinical studies
CMC (Chemistry, Manufacturing, and Controls)
Clinical Development for New Drug
Development of a regulatory strategy roadmap specialized in clinical medicine
Competent MDs and PMs
Clinical development operation based on medical and clinical development knowledge
Individual clinical trial operation
: Conducting clinical trials in compliance with regulatory agency/IRB regulations
Success in Clinical Development
Drug Approval (NDA/BLA)
CRO & Trial Management
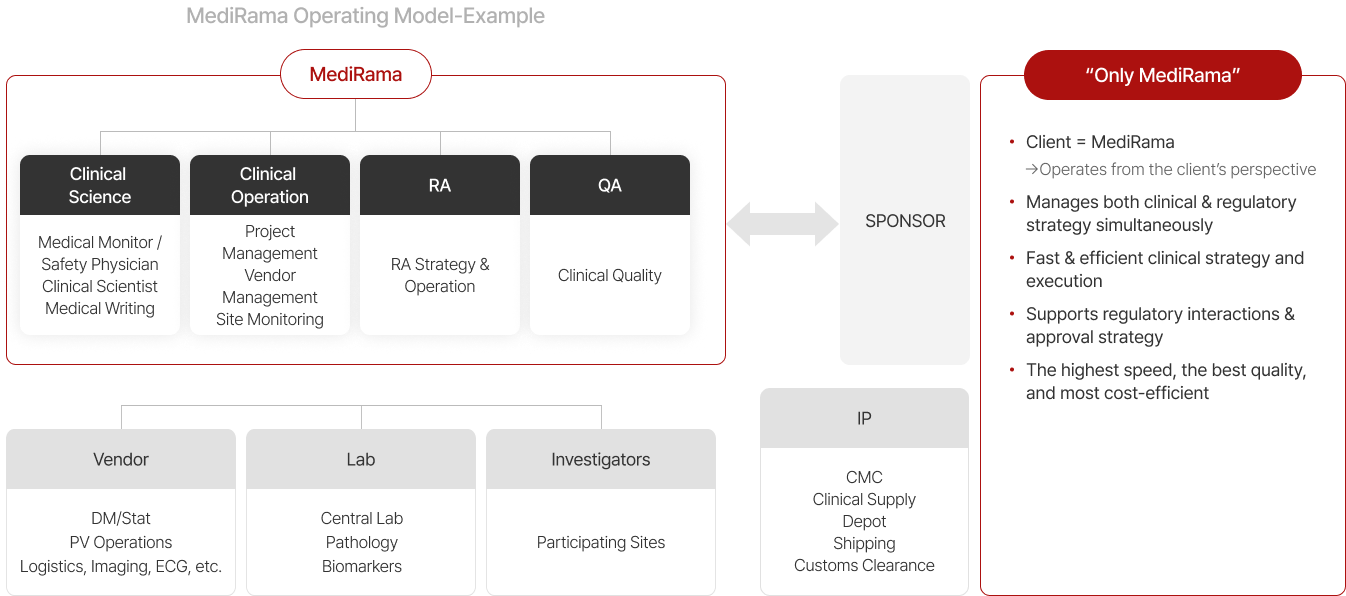
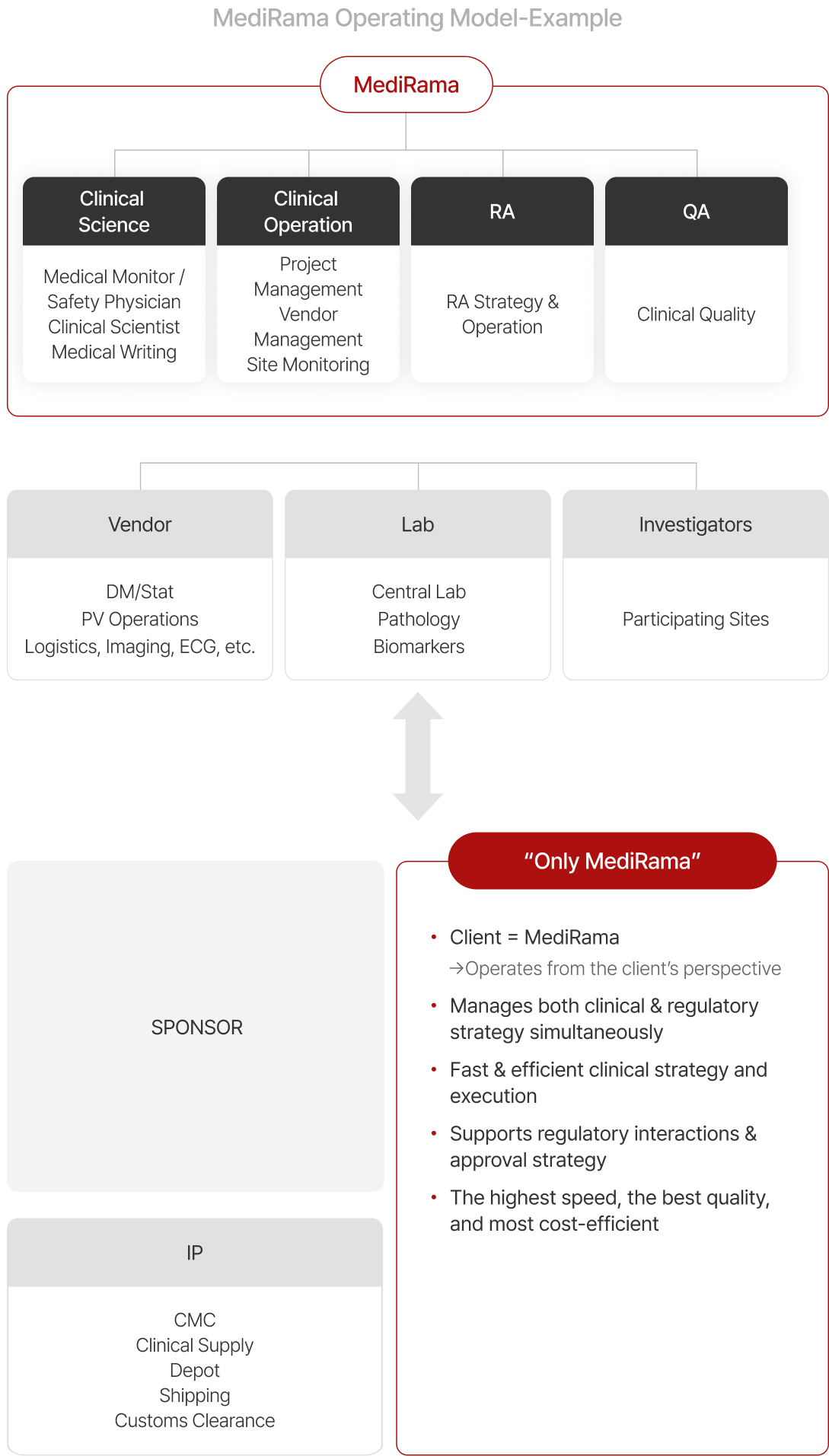
Commercial Consulting

MediRama: Clinical Experts for Commercial Success
- MediRama is a team of clinical development experts specializing in strategic trials and regulatory pathways to accelerate drug approval and commercialization.
We help maximizing the asset value and driving successful business growth for drug developers.
-
R&D
Is your R&D/portfolio strategy aligned with your company's vision and business strategy?
-
Licensing
Can this actively developed asset be successfully out-licensed?
-
Business
Finding partners for global markets—but what’s the strategy for domestic business?
Corporate Portfolio Strategy Consulting
Strategic Portfolio Assessment & Planning through
Current portfolio composition and future therapeutic focus
Commercial capabilities and differentiation from competitors
Alignment of development/acquisition pipeline with the existing portfolio
Go/No-Go decisions for development and in-licensing projects
Comprehensive Support for Licensing Deals
Strategy & Analysis: Business development strategy, market & competitive analysis, valuation
Partnering: Screening, shortlist creation, BD deck development
Execution: Partnering events, product pitching, negotiations, contract support
Business Strategy for the Domestic Market
Market access, launch, distribution & promotion, PR, alliance, etc.
