Resources
[GCCL Special Contribution ②] The Significance of the FDA’s Oncology Expedited Review System and Its Strategic Utilization
- Writer:관리자
- Date:2025-09-23
- Source:THE BIO

On the 17th, a webinar co-hosted by Medirama and GCCL attracted strong interest, with over 550 people registering in advance. During the event, Hanlim Moon, CEO of Medirama, gave a lecture titled “FDA’s Oncology Expedited Programs in Action: A Case-Based Approach to Strategic Drug Development.” The Bio introduces the details of his lecture through a two-part series of contributed articles. This is the second installment, following the first (see Part 1).
◇ Lessons Learned from Case Studies
④ Columvi (ingredient: glofitamab): Highlighting the Importance of Diversity
Roche’s glofitamab, a CD20/CD3 bispecific antibody, received accelerated approval as a treatment for patients with relapsed or refractory B-cell lymphoma, drawing attention as a new therapeutic option. However, the U.S. Food and Drug Administration’s (FDA) Oncologic Drugs Advisory Committee (ODAC) recently issued a negative recommendation after reviewing Phase 3 clinical data. The key issue was not simply the magnitude of efficacy but whether the data could truly represent the U.S. patient population.
The ODAC vote ended in an overwhelming 8-to-1 rejection, eliciting mixed reactions in the market. Media reports described the outcome with words such as “concern,” “disappointment,” and “stricter than expected evaluation.”
-
Concern referred to the lack of representativeness in the data, as pointed out by the FDA.
-
Disappointment reflected the dashed hopes of Roche and investors regarding glofitamab’s approval prospects.
-
Stricter than expected described the evaluation stance of the ODAC.
Ultimately, this decision was not just the failure of a single new drug—it starkly illustrated the consequences of failing to ensure representativeness of U.S. patients in global clinical development.
In fact, more than half of the study population was enrolled in Asia, while only about 10% of patients came from North America. More importantly, glofitamab’s therapeutic superiority was not observed at all in the subgroup of non-Asian patients.
In other words, while the overall survival analysis of the entire study population showed very favorable results (25.5 months vs. 12.9 months), when looking specifically at U.S. or Caucasian patients, no survival benefit was observed. This was precisely the point of concern raised by the FDA (see Figures 1 and 2 below).
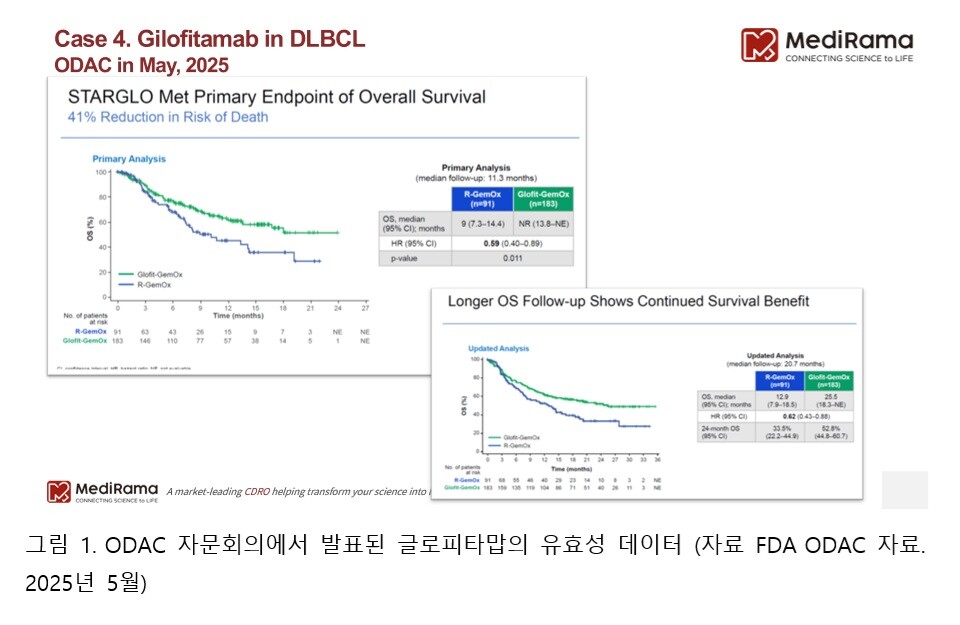
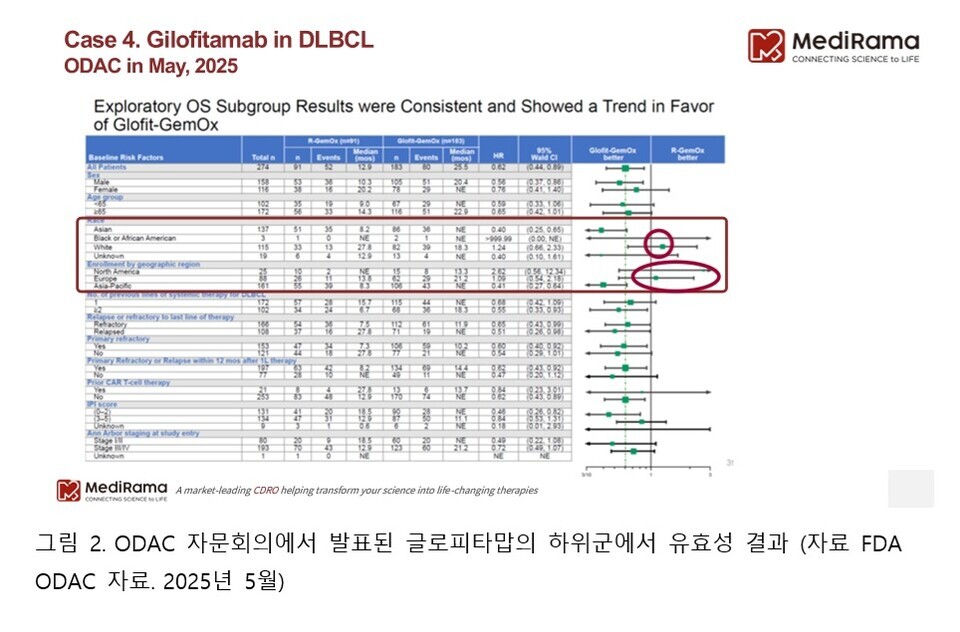
Ultimately, the question raised by ODAC was not simply “Does this drug work?” but rather “Can these results be generalized to U.S. patients?” To gain approval in the U.S. market, reliable clinical evidence based on domestic patient populations and racial representativeness is a prerequisite. Glofitamab stands as a vivid example of this principle.
◇ Conclusion
The FDA’s expedited review programs serve as a powerful catalyst in oncology drug development, but an approach that prioritizes only speed can instead lead to failure. For success, four elements are essential:
-
Close collaboration with the FDA from the early stages,
-
Strategic use of multiple expedited programs,
-
Thorough preparation for confirmatory clinical trials, and
-
Ensuring diversity among patient populations.
In the end, the decisive factor in the success or failure of these programs is not the system itself but the developer’s strategic insight and the strength of the scientific evidence. To provide patients with treatments that are not only faster but also safe and reliable, balance between speed and rigor is indispensable.
This contribution underscores that the essence of the FDA’s expedited review programs lies not merely in acceleration but in striking the right balance between scientific evidence and patient access. It serves as a reminder that the strategic use of these programs can be the critical turning point determining success or failure in anticancer drug development.
Source: The Bio (https://www.thebionews.net)
